Chemistry Unit
We had a blast doing Chemistry this year to help you plan your own chemistry unit I am going to include an outline below as well as some of the resources we used and loved. I learned a lot by building this unit and changed the outline to reflect how I would do things differently going forward. We met once a week for 2 hours each lesson below is an outline for one week’s worth of lessons/labs. These outlines are to inspire scope & sequence as you plan your own class. Feel free to add ideas in the comments below to help others as they plan their upper elementary chemistry class.
Broad Outline:
Introduction to Chemistry and the Scientific Method
Phases of Matter Review
Elements Atoms and Molecules
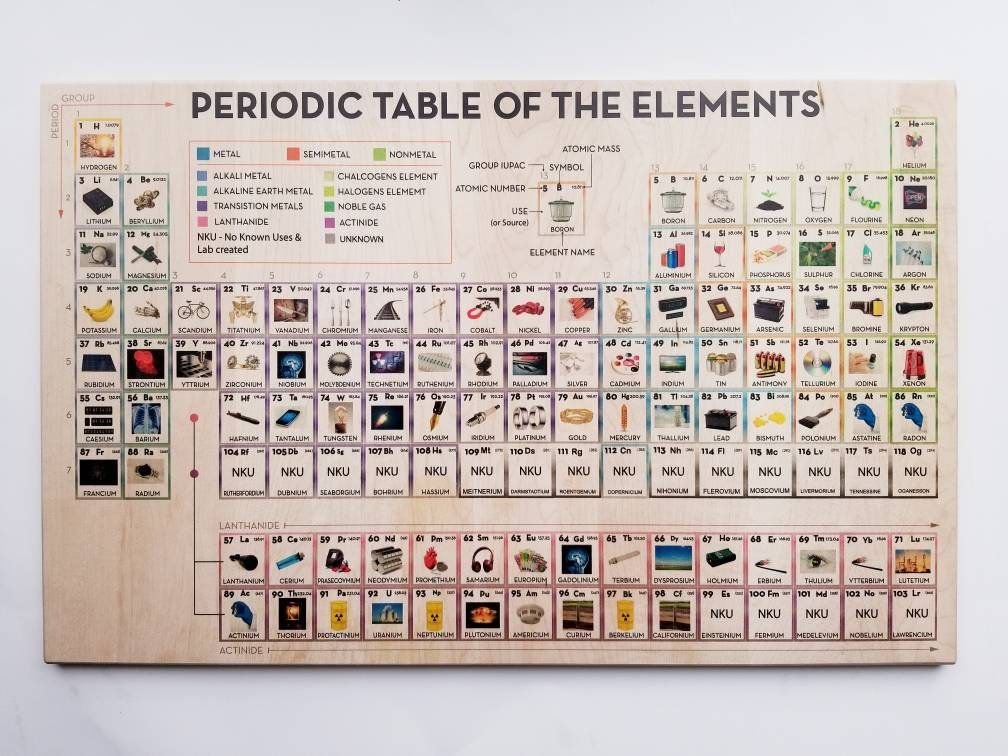
The Atomic Structure and Periodic table (the organization of the elements)
Element Reports
Mixtures and Pure Substances
Crystals (Boron Elements)
What is Air (Nitrogen Elements, Halogens)
Molecules, Compounds, and Chemical Bonding
Molecule Building
Unique Properties of Water
Chemical and Physical Changes
Oxidation (Transition metals, Oxygen Elements)
Acids & Bases
Large Chemical Reactions (Alkali Metals)
Alkaline Earth Metals (Fireworks)
Organic Chemistry (Carbon Elements)
DNA & RNA
Marie Curie (Lanthanides & Actinides)
Element Game
Favorite Resources:
Click the images for links.
Extended Outlines
I may one day expand on this with step-by-step instructions, video and experiment links, etc. but for now, I hope this helps you as you put your plan together.
Week#1 Introduction to Chemistry and the Scientific Method
What is Chemistry
Safety Equipment and Practices
Review of Measuring
Scientific Method
Chemist: Mikhail Tsvet bio
Lab: Chromatography with markers, and coffee filters
Week #2 Phases of Matter Review
“What is the World Made of?” by Kathleen Weider Zoehfeld
States of Matter video demo
Glue solids, liquids, and gas poster in the science notebook
Science Demo of solids, liquids, and gases
Changes in the state (melting, evaporating, condensation, sublimation) with demo
Water cycle review with mat
Effects of Pressure on states of matter
Dry ice fun
Chemist: Jabir Ibn Hayyan's bio
Week#3 Elements, Atoms, and Molecules
Magic School Bus Molly Cule Episode
Define Elements with legos
Define Atoms and their parts
Chemist: Maria Goppert-Mayer bio
Make an edible model of an atom
Week #4 Atomic Structure and the Periodic Table
Review Subatomic particles
Chemist: Neils Bohr bio
Orbitals from Theodor Grey’s Molecules book
Bohrs model of the atom with Mirus Toys
Chemist: Dmitri Mendeleev's bio
Mendeleev’s Organization
Build a periodic table with Mirus Toys
Element Families with The Lesson Hub
Week #5 Element Reports
Element Reports
Build Models of Atoms
Explore the table of the elements/ Elements book
Week #6 Mixtures and Pure Substances
Define Mixtures, solutions, and pure substances
Make lunch that has a mixture and a solution
A salad for a mixture
Lemonade for a Solution
Separating Mixtures
Evaporation
Filtration
Chemical & Physical properties
Sorting game
Week #7 Crystals
What are Crystals?
Chemist: Margaret Cairns Etter bio
Boron Elements
Define, and color the periodic table
Lab: Boron crystals
Open Geodes
Particulate is a sign of Chemical Change
Lab: Milk & Coke-a-cola experiment
Week #8 What is Air?
Elements found in the air we breathe
Air has mass
Air density
Lab: Helium car experiment
Plant Respiration vs Human Respiration
Demo: Plant respiration underwater or in a spinach container
Nitrogen Elements
Define
Color periodic table
Halogens
Define
Color Periodic Table
Go downtown to see neon lights and identify different halogens used for the different colors
Week #9 Molecules, Compounds, and Chemical Bonding
Molecules, compounds, and ions review
Types of Bonds
Covalent, Ionic, and metallic bonding
Polar vs Nonpolar
Noble Gases
Define
Color in the periodic table
How to graph a chemical bond
Week #10 Molecule Building
Happy Atoms Set
Molecule Book
Week #11 Unique Properties of water
Water as a solvent
Dissolve candy
Surface tension
Floating paper clips
Pond Skitters walking on water video
Attractive properties of water
Cohesion (water dropper and penny)
Adhesion (make a star out of toothpicks and drop water until it sticks to each other and the toothpicks)
Less dense as solid than liquid
Ice floats in water…but why? (Critical thinking question)
Chemist: Anges Pockels bio
Week# 12 Chemical and Physical Changes
Review Chemical and Physical Properties
Physical vs Chemical Changes
Physical changes
Break up cookie
Chemical changes
Make cookies
Chemist: Joseph Priestly bio
Types of Reactions
Synthesis
Decomposition
Combustion
Single Replacement
Double Replacement
Law of Conservation of Mass memory work
Week #13 Oxidation
Transition Metals
Define
Color periodic table
Oxygen Elements
Define
Color periodic table
Why do transition metals react so often with oxygen?
Chemist: Antoine Lavoisier's bio
Oxidation Experiment
Turn a penny green
Statue of liberty video
Catalysts and Inhibitors
Define
Talk about applying zinc to iron, painting metal, etc. to slow rust vs how in wet places like near the ocean things rust faster since water is a catalyst.
Week #14 Acids & Bases
Review chemical reaction evidence
Review law of conservation of mass
Chemist: Svante August Arrhenius bio
Define acids and bases
Lab: Baking soda and vinegar experiment
Chemist: S.P.L. Sorensen bio
PH scale
Salt Ions
Week #15 Large Chemical Reactions
Alkali Metals
Define
Watch videos of reactions to water
Color on the periodic table
Acids and Bases Review
WHY are they so reactive?
Nuclear Bombs
How they are made, why they are so violent
Lab: Elephant Toothpaste experiment
identify the acid, base, catalyst, and product in the reaction
Week #16 Alkaline Earth Metals
Catch up on Element families on the periodic table if you missed coloring any so far
Alkaline earth metals
Define
Color in the periodic table
Fireworks history and display (or video)
Optional time filler: Magic School Bus: Ready, Set, Dough episode
Week #17 Organic Chemistry
Define organic chemistry
Atoms to humans (atom, element, molecule, compound, cell, tissue, organ, being)
Map it out in the science notebook cut/glue activity
Biomolecules (macronutrients)
Carbohydrates
Quick energy Source
Lipids
Long term energy Source
Define Hydrophobic
Proteins
Building blocks, enzymes, etc
Nucleic Acids
DNA & RNA
Chemist: Gerty Cori bio
Lab: Cori Cycle Experiment
Alcohols & Esters
Happy Atom Set
Hydrocarbons
Often used as fuels, colorless, odorless, and combustible!
Teach kids how to identify a gas leak and the importance of carbon dioxide detectors as some harmful gases are not detectable to our senses.
Bonus: Fun with bubbles
How bubbles work
Week #18 DNA & RNA
Review biomolecules
Define DNA & RNA
Make a model of DNA
Gumdrops and toothpicks
Lab: Milk into “plastic” experiment
Polymers and Plastics
Pencils into a plastic bag full of water demo
Week #19 Marie Curie
Documentary or biography
Define Radioactivity
Half-Life demo with cereal being divided in half and eaten every 1 minute.
Lanthanides and Actinides
Define
Color on the periodic table
Week #20 Element Game
Play Periodic and Ion games for review
Recite memory work
Get a Free printable outline of the unit here:
Pinable image for the Chemistry unit